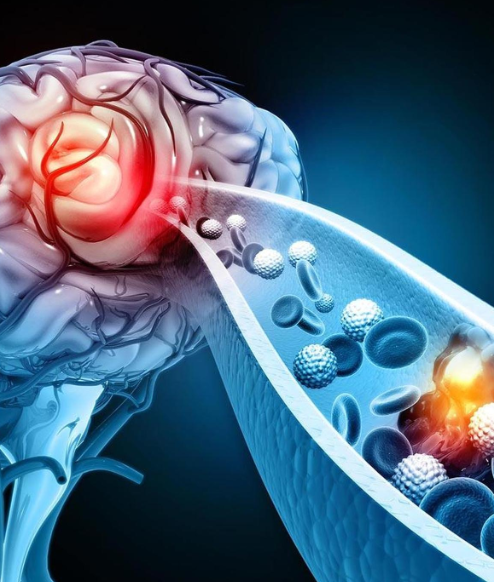
The device is used to treat severe strokes by removing clots and restoring blood flow to the brain within 24 hours
The indigenously developed Supernova stent retriever—approved in India using only domestic clinical trial data— is expected to be manufactured in the country and launched in February 2026. The device is used to treat severe strokes by removing clots and restoring blood flow to the brain within 24 hours.
Developed by Gravity Medical Technology, its clinical trial was led by the All-India Institute of Medical Sciences (AIIMS), Delhi. The Central Drugs Standard Control Organisation (CDSCO) has approved the device’s manufacturing and marketing in India after the trial demonstrated good safety and efficacy outcomes. It is the first time a stroke device has been approved in India based solely on domestic clinical trial data.